Abstract
Introduction:
Large granular lymphocytic leukemia (LGLL) is a rare, indolent malignancy arising from cytotoxic T cells (T-LGLL, ~85%) or NK cells [chronic NK-cell lymphoproliferative disorder (CLPD-NK), ~15%]. When treatment is needed, single-agent immunosuppressive therapeutics are usually used, including low-doses of methotrexate (MTX), cyclophosphamide (Cy), and cyclosporine A (CSA). The coexistence of LGLL with autoimmune diseases (ADs) especially rheumatoid arthritis (RA) and Felty Syndrome (FS) has been reported in some patients (pts), suggesting a role of initial strong antigenic stimuli from autoantigens in the pathogenesis of LGLL. However, it remains unclear whether AD-associated LGLLs have unique clinical features or treatment outcomes. We have recently published the largest retrospective study of 319 LGLL cases (Dong et al. 2021). Here we report the outcomes of the LGLL pts with coexisting ADs.
Methods:
All patients who presented to Moffitt Cancer Center from 2001 to 2020 with a diagnosis of coexistence of T-LGLL or CLPD-NK and AD were included. Diagnostic criteria, treatment and response evaluation were reported elsewhere (Dong et al. 2021). Comparison of response rates was performed using Fisher's exact test or Chi-square test when appropriate. Median survival was estimated using Kaplan-Meier method and compared with log-rank test. Multivariate analysis was not done due to limitation of sample size. All analyses were done using SAS 9.4.
Results:
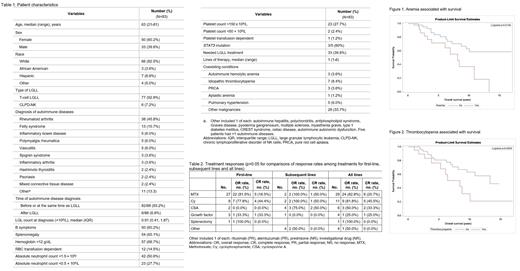
The patient characteristics are listed in Table 1. Among the 83 pts, 77 (92.8%) had T-LGLL and 6 (7.2%) had CLPD-NK. The most common ADs were RA [38 (45.8%)], followed by FS 13 (15.7%), and inflammatory bowel disease, polymyalgia rheumatica and vasculitis [5 (6%) each]. 33 (39.8%) pts needed treatment for LGLL. Three of the 5 pts who underwent NGS testing were positive for STAT3 mutation.
In our practice, we favor MTX as the frontline therapy for LGLL with coexisting ADs such as RA. The treatments and responses are presented in Table 2. Among the 29 pts who received MTX, 24 (82.8%) had response, including 6 (20.7%) complete response (CR). Among the 11 pts treated with Cy, 9 (81.8%) had response and 5 (45.5%) had CR. CSA was used in 6 pts and had response in 3 (50.0%) and CR in 2 (33.3%). The response rates were not different between MTX, Cy and CSA, neither were the CR rates, although the statistical testing was limited by sample size. Consistent with our previous findings in LGLL, the response to growth factor (GCSF) was low and only 1 of 4 pts responded. Compared to the 131 LGLL pts without co-existing AD in our series, AD-associated LGLL had higher response rates to MTX, Cy or CSA (61.9% vs 78.3%, respectively, p=0.04).
Interestingly, pts with CLPD-NK were less likely to respond to CSA, MTX or Cy compared to pts with T-LGLL [1/5 (20%) vs 35/41 (85.4%), p=0.006]. The type of AD, age, splenomegaly, anemia, neutropenia, thrombocytopenia or STAT3 mutation were not associated with response.
With a median follow-up of 5.2 (IQR 2.0-9.5) years, the median survival was 10.9 (95% CI 8.1-not estimable) years. Anemia and thrombocytopenia were associated with worse survival (figures 1 and 2). The median survival for pts with vs without anemia was 8.1 (95% CI 3.9-not estimable) years vs not estimable (95% CI 8.7-not estimable), p=0.01. The median survival for pts with vs without thrombocytopenia was 8.4 (95% CI 3.8-10.1) years vs 13.3 (95% CI 8.1-not estimable) years, p=0.05. The type of LGLL, blood LGL count, splenomegaly, or neutropenia were not associated with survival.
Conclusions:
The majority (~80 %) of LGLL pts with AD responded to MTX, Cy or CSA, although CR rate was low (20-45%). Interestingly, CLPD-NK pts were less likely to respond compared to T-LGLL which could be attributed to a small sample size and warrants an expanded case study. Anemia and thrombocytopenia were associated with worse survival. Future studies will be of interest to evaluate the hematological responses of LGLL in patients with coexisting ADs treated with FDA-approved biological agents for AD such as TNF inhibitors, IL-6 antagonists and T-Cell co-stimulation blocker. Such studies may provide insights into LGLL treatment as both diseases may share some common pathogenic pathways.
Sokol: Kyowa-Kirin: Membership on an entity's Board of Directors or advisory committees; Dren Bio: Membership on an entity's Board of Directors or advisory committees.
Rituximab and alemtuzumab have not been approved by FDA for LGL leukemia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal